Plants have a unique method of influencing the wetting properties of their surfaces by controlling the composition and morphology of exterior wax layers excreted from the plant surfaces. In this way nature has been able to use one material to achieve both the hydrophobic nature and the high degree of roughness needed to achieve a superhydrophobic wetting state. Superhydrophobicity is of significant interest as it has many applications which range from self-cleaning surfaces, and anti-icing surfaces, to drag reduction and antifouling.
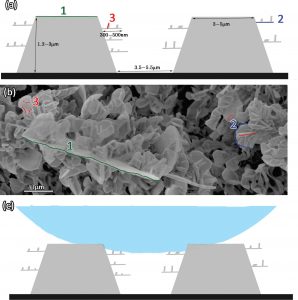 Figure 1 – (a) Schematic illustration of the hierarchical surface created via thermal deposition of paraffin wax. Numbers 1-3 indicated on the illustration match the corresponding features in (b). (b) HR-SEM image of said fluorinated wax structures. (c) Schematic illustration of a liquid drop on top of the hierarchical wax structures.
Figure 1 – (a) Schematic illustration of the hierarchical surface created via thermal deposition of paraffin wax. Numbers 1-3 indicated on the illustration match the corresponding features in (b). (b) HR-SEM image of said fluorinated wax structures. (c) Schematic illustration of a liquid drop on top of the hierarchical wax structures.
Our lab has studied these biological systems to get inspiration for fabrication methods and engineering applications of single-component superhydrophobic surfaces. Using thermally evaporated paraffin waxes deposited on various substrates we showed that an increase of the hydrophobicity occurs over time until a superhydrophobic state is achieved. This is was shown to occur via the self-assembly of the paraffin waxes at room temperature into rough hierarchical surfaces (1, 2). Fluorinated paraffin waxes were also used in a similar method to create hierarchical surfaces exhibiting both superoleophobic and superhydrophobic properties in tandem with exceptional surface stability (3). This fabrication method can be easily utilized in a variety of applications where either a superhydrophobic, superoleophobic, or both are needed.
Furthermore the liquid-surface interface of a sessile drop in a superhydrophobic state was studied using direct imaging via confocal microscopy (4). This is the first time the shape of the water-air interface bellow a drop on a superhydrophobic surface was imaged directly and that the curvature of the water-air interface was shown to be constant and close to zero for liquids of varying density and surface tension.
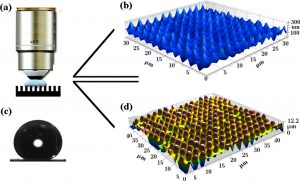 Figure 2 – Imaging the water-air interface beneath a water drop on a superhydrophobic surface. (a) Macro view of the confocal immersion lens inside the water drop, (b) 3D image of the water-air interface, (c) water drop demonstrating a 170˚ contact angle indicating a superhydrophobic state, (d) 3D image of the microtextured surface (4).
Figure 2 – Imaging the water-air interface beneath a water drop on a superhydrophobic surface. (a) Macro view of the confocal immersion lens inside the water drop, (b) 3D image of the water-air interface, (c) water drop demonstrating a 170˚ contact angle indicating a superhydrophobic state, (d) 3D image of the microtextured surface (4).
We further developed multifunctional superhydrophobic coatings comprised of non-toxic saturated fatty acids (SFAs). These molecules are naturally present in biological systems, including human body, and are a part of human daily dietary uptake. The latter makes fatty acids promising candidate compounds to serve as a coating agent for various applications where strict safety regulations apply, such as food contact surfaces, agricultural, biomedical etc. In contrast to paraffin waxes, fatty acids contain a terminal carboxylic group, which may be involved in their antibacterial activity and can, therefore, affect the coating's properties as well. Moreover, fatty acids are known as natural antimicrobial agents, which makes them even more advantageous for application in functional coatings due to the synergetic effect of their intrinsic superhydrophobic and antimicrobial properties.
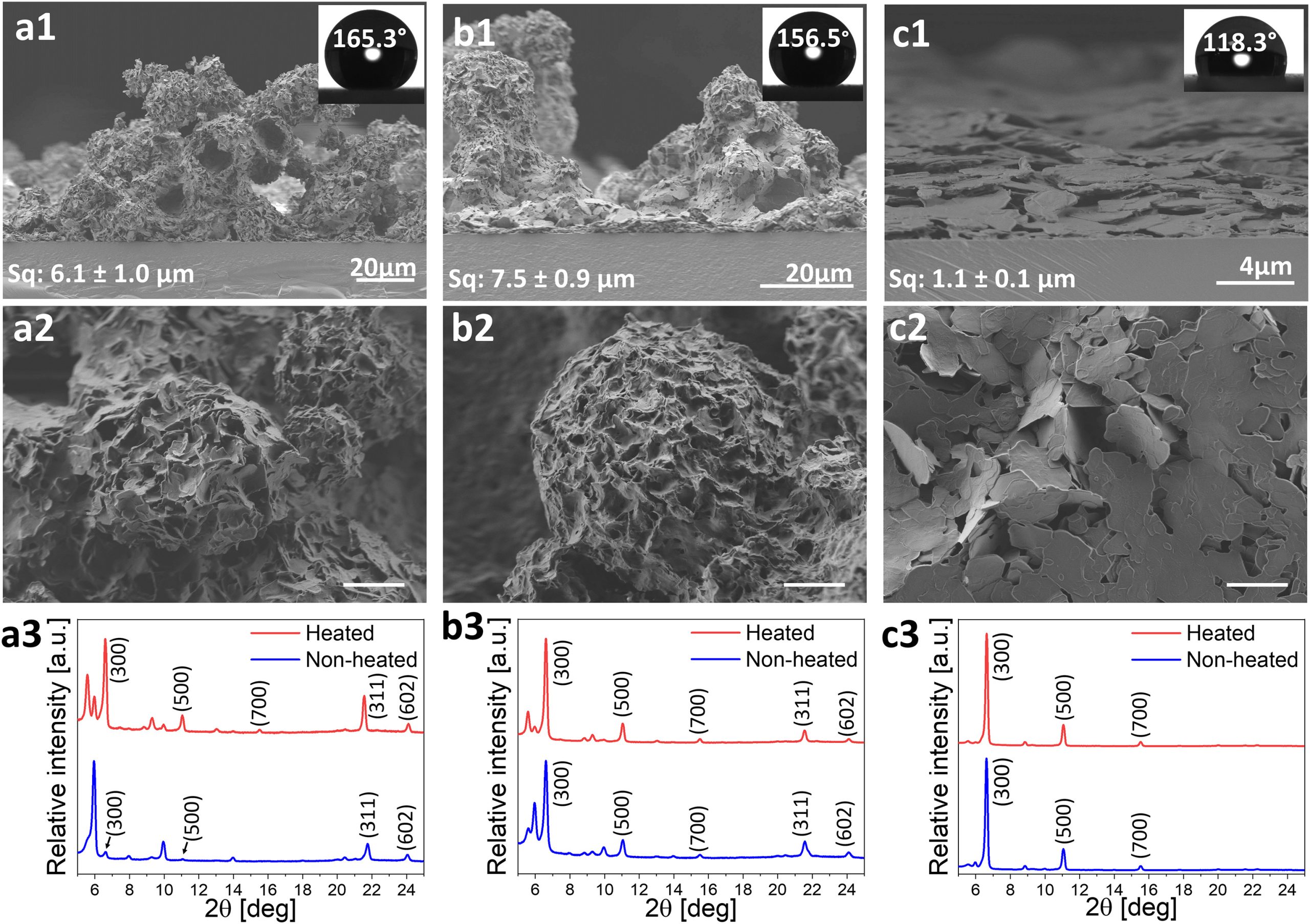
We have successfully established a facile applicative and scalable deposition method to form superhydrophobic coatings using various SFAs utilizing a spraying technique. Planar and cross-sectional imaging of the spray-deposited coatings revealed that in the case of diethyl ether- and acetone-based stearic acid (18C) coatings the surface is covered with sphere-shaped aggregates that are tens of microns in size and composed of smaller micron-sized crystals.
 Very recently, we have expanded this concept into the plant realm. By applying it onto plant leaves, which naturally exhibit hydrophilic surface properties, fatty acids-based coating renders the plant surface superhydrophobic anti-adhesive and self-cleaning properties to serve for multifunctional unti-fungul plant protection. This research has received funding from the Horizon Europe for Research and Innovation program under grant agreement No.101099462 and is currently in progress in the frame of the SafeWax Consortium. SafeWax technology will be demonstrated on grapevines, which are severely damaged by fungal-associated diseases.
Very recently, we have expanded this concept into the plant realm. By applying it onto plant leaves, which naturally exhibit hydrophilic surface properties, fatty acids-based coating renders the plant surface superhydrophobic anti-adhesive and self-cleaning properties to serve for multifunctional unti-fungul plant protection. This research has received funding from the Horizon Europe for Research and Innovation program under grant agreement No.101099462 and is currently in progress in the frame of the SafeWax Consortium. SafeWax technology will be demonstrated on grapevines, which are severely damaged by fungal-associated diseases.Publications:
Prudnikov E, Polishchuk I, Sand A, Abu Hamad H, Massad-Ivanirn N, Segal E and Pokroy B. Self-assembled fatty acid crystalline coatings display superhydrophobic antimicrobial properties. Mater Today Bio, 2022, 18:100516.
Rich B and Pokroy B. A study on the wetting properties of broccoli leaf surfaces and their time dependent self-healing after mechanical damage. Soft Matter 2018; 38:7773. HIGHLIGHTED ON THE COVER.
Pechook S et al. and Pokroy B. Bio-Inspired Superoleophobic Fluorinated Wax Crystalline Surfaces, Adv Funct Mater 2013, 23: 4572. HIGHLIGHTED ON THE COVER.
Pechook S and Pokroy B. Self‐Assembling, Bioinspired Wax Crystalline Surfaces with Time‐Dependent Wettability, Adv Funct Mater 2012, 22:745-750.
Pechook S and Pokroy B. Bioinspired hierarchical superhydrophobic structures formed by n-paraffin waxes of varying chain lengths, Soft Matter 2013, 9:5710.
Haimov B, et al. and Pokroy B. Shape of Water–Air Interface beneath a Drop on a Superhydrophobic Surface Revealed: Constant Curvature That Approaches Zero. J Phys Chem C 2013, 117:6658.



